PRELIMINARY STUDY ON PERFORMANCE OF Zn-DOPED ZEOLITE IN LOW-TEMPERATURE CO2 ADSORPTION
DOI:
https://doi.org/10.22452/mjs.vol43sp1.6Keywords:
Zeolite, CO2 adsorption, adsorption capacity, low temperatureAbstract
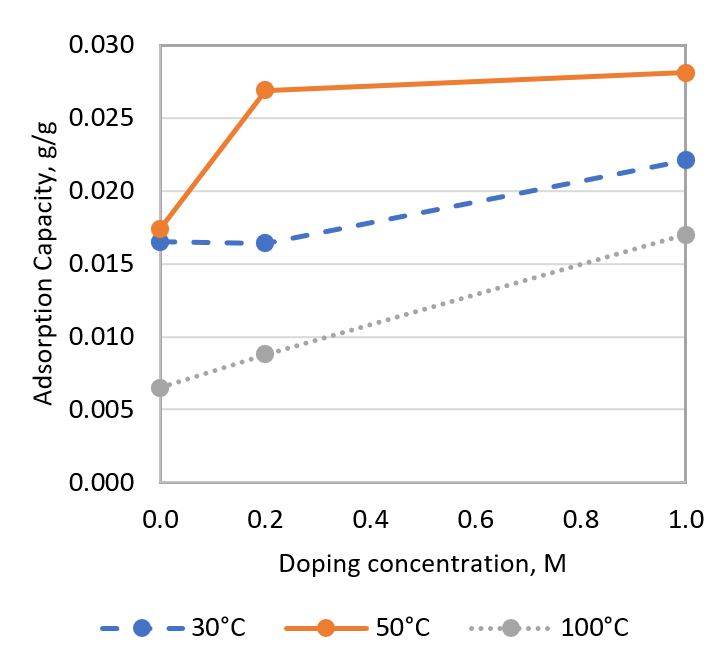
Zeolite has been identified as a potential low-temperature CO₂ adsorbent with the highest adsorption capacity among adsorbents in its category. However, its adsorption capacity remains relatively low, limiting its industrial application for CO₂ adsorption. Additionally, there is a need to increase the optimal adsorption temperature of this porous material to effectively adsorb CO₂ emitted from flue gas, which has an average temperature of 100 - 125°C. To address these challenges, a preliminary study on Zn-doped zeolite has been conducted. This study aims to investigate the ability of Zn-doped zeolite to enhance CO₂ adsorption capacity and its effect on the optimal temperature for CO₂ adsorption. Zinc-doped zeolite was synthesized by doping zinc oxide into natural zeolite using a zinc ion exchange method at different doping concentrations (0.2 M & 1.0 M). Undoped natural zeolite was studied as a benchmark. Their CO₂ adsorption performance was tested using TGA at 30°C, 50°C, and 100°C. The effects of temperature and doping concentration on adsorption capacity were investigated. The adsorbent samples were characterized using X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) with Energy Dispersive X-ray (EDX) analysis. It was found that increasing the temperature from 30°C to 50°C increased the CO₂ adsorption capacity, but the capacity decreased when the temperature was further increased to 100°C. Furthermore, increasing the doping concentration tended to enhance the CO₂ adsorption capacity. The highest adsorption capacity of 0.0281 g CO₂/g sorbent was observed in zinc-doped zeolite with a 1.0 M doping concentration at 50°C. The improvement was mainly attributed to the zinc oxide doped on the zeolite, which provided a functional group that formed chemical bonds with CO₂. This study also found that the adsorption rate of CO₂ was predominantly influenced by temperature, while the effect of doping concentration was less significant. All testing and characterization results suggested that the zinc-ion exchange method improved the CO₂ adsorption capacity of zeolite.
Downloads
References
Bezerra, D. P., Silva, F. W. M. da, Moura, P. A. S. de, Sousa, A. G. S., Vieira, R. S., Rodriguez-Castellon, E., & Azevedo, D. C. S. (2014). CO2 adsorption in amine-grafted zeolite 13X. Applied Surface Science 314:314–321.
Chen, C., Yu, J., Song, G., & Che, K. (2023). Desorption performance of commercial zeolites for temperature-swing CO2 capture. Journal of Environmental Chemical Engineering 11(3): 110253.
Fu, D., Park, Y., & Davis, M. E. (2022). Zinc Containing Small-Pore Zeolites for Capture of Low Concentration Carbon Dioxide. Angewandte Chemie International Edition 61(5): e202112916.
Gankanda, A., Cwiertny, D. M., & Grassian, V. H. (2016). Role of Atmospheric CO2 and H2O Adsorption on ZnO and CuO Nanoparticle Aging: Formation of New Surface Phases and the Impact on Nanoparticle Dissolution. The Journal of Physical Chemistry C 120(34): 19195–19203.
Hagen, J. (2015). Industrial Catalysis: A Practical Approach. 3rd ed. New Jersey: John Wiley & Sons.
Hauchhum, L., & Mahanta, P. (2014). Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. International Journal of Energy and Environmental Engineering 5(4): 349-356.
Huong, P.-T. & Lee, B.-K. (2017). Improvement of selective separation of CO2 over N2 by transition metal–exchanged. Microporous and Mesoporous Materials 241: 155-164.
Jha, B. & Singh, D. N. (2016). Advanced Structured Materials. 1st ed. Singapore: Springer.
Klugmann-Radziemska, E. (2022). 9.10 - The Environmental Benefits of Photovoltaic Systems: The Impact on the Environment in the Production of Photovoltaic Systems: With a Focus on Metal Recovery. In T. M. Letcher (Ed.), Comprehensive Renewable Energy (Second Edition), pp. 140–151. Oxford: Elsevier.
Kusumastuti, R., Sriyono, Pancoko, M., Butar-Butar, S-L., Putra, G.E., and Tjahjono, H. (2019). Study on the mechanism of CO2 adsorption process on zeolite 5A as a molecular sieve in RDE system: An infrared investigation. Journal of Physics: Conference Series 1198: 032009.
Munawar, K., Mansoor, M.A., Olmstead, M.M., Zaharinie, T., Zubir, M.N.M., Haniffa, M., Basirun, W.J., & Mazhar, M. (2020). Fabrication of Ag-ZnO composite thin films for plasmonic enhanced water splitting. Materials Chemistry and Physics 255: 123220.
Pham, T.-H., Lee, B.-K., & Kim, J. (2016). Novel improvement of CO2 adsorption capacity and selectivity by ethylenediamine-modified nano zeolite. Journal of the Taiwan Institute of Chemical Engineers 66: 239-248.
Qian, X., Ren, Q., Wu, X., Sun, J., Wu, H., & Lei, J. (2018). Enhanced water stability in Zn-doped zeolitic imidazolate framework-67 (ZIF-67) for CO2 capture applications. Chemistry Select 3: 657.
Rajakrishnamoorthy, P., Saravanan, C.G., Natarajan, R., Karthikeyan, D., Sasikala, J., Josephin, J.S.F., Vikneswaran, M., Sonthalia, A., & Varuvel, E.G. (2023). Exhaust emission control of SI engines using ZSM-5 zeolite supported bimetals as a catalyst synthesized from coal fly ash. Fuel 340: 127380.
Sharma, K., Park, Y-K., Nadda, A.K., Banerjee, P., Singh, P., Raizada, P., Banat, F., Bharath, G., Jeong, S.M., & Lam, S.S. (2022). Emerging chemo-biocatalytic routes for valorization of major greenhouse gases (GHG) into industrial products: A comprehensive review. Journal of Industrial and Engineering Chemistry 109: 1-20.
Zhang, J., Huang, D., Shao, J., Zhang, X., Zhang, S., Yang, H., & Chen, H. (2023). A new nitrogen-enriched biochar modified by ZIF-8 grafting and annealing for enhancing CO2 adsorption. Fuel Processing Technology 231: 107250.
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





