A Comparative Study of Various Extraction Techniques for Extracting Antioxidant-Rich Phytoconstituents from Eryngium foetidum Leaves Utilizing Spectrophotometric and HPLC Applications
Keywords:
: Antioxidants, extraction methods, Eryngium foetidum, HPLC analysis, phytochemicals, spectrophotometric analysisAbstract
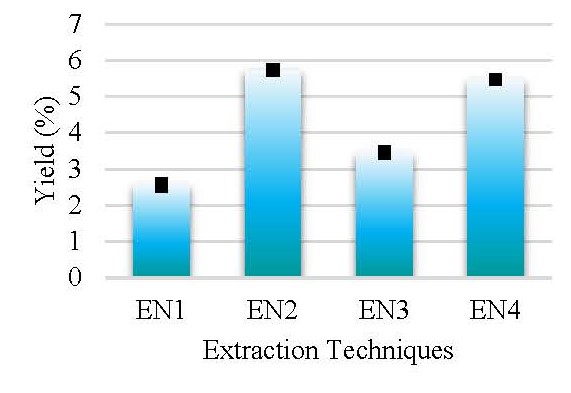
Eryngium foetidum L. (Apiaceae) is known to possess many healthcare properties and has been used in the traditional system of medicine for various health issues. Despite that less scientific data on its photochemistry and antioxidant properties is limited. Therefore, this study aimed to document the photochemistry and antioxidant properties of leaves by following different extraction techniques to extract the plant constituents. Sonication (EN1), Soxhlet (EN2), maceration (EN3), and maceration with heat (EN4) were used as the extraction techniques while water was used as the extracting solvent. The HPLC method with a PDA detector was developed to compare the phytochemicals profile under each technique. The antioxidant capacities and content of saponins (SC), terpenoids (TC), flavonoids (TFC), tannins (TTC), alkaloids (AC), and polyphenolics (TPC) were determined spectrophotometrically. The EN2 and EN4 methods were identified using the HPLC-PDA application as yielding the highest overall results and giving a wide range of phytochemicals. The quantitative analyses resulted in high SC, TTC, TC, and TPC in the EN4 extraction process (185.84±0.54 mg SE/g, 36.99±0.64 mg TAE/g, 0.89±0.01 mM LE/g, and 37.37±0.65 mg GAE/g, respectively) and low in the EN1. TFC levels in EN2 were high (11.84±0.14 mg QE/g), whereas it was low in EN3. Furthermore, AC was higher in the extraction method EN3 (1.67±0.01 mg AE/g) and lower in the extraction technique EN2. The total antioxidant capacity was higher in the EN4 extract (47.17±0.20 mg Trolox Eq/g) and lower in the EN1 extract. The lowest IC50 in the 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) assay was noted for EN3 extract (12.91±0.02 mg/mL) revealing the highest scavenging activity than the other extracts. Based on HPLC and spectrophotometric analyses, maceration with heat (EN4) is recommended for efficiently extracting polyphenols and antioxidants from E. foetidum leaves. The application of heat would also improve the extraction efficiency of phytochemicals.
Downloads
References
Abeysuriya, H. I., Bulugahapitiya, V. P., & Jayatissa, L. P. (2021). Comparative account of vitamin C contents, antioxidant properties and iron contents of minor fruits in Sri Lanka. Plant Science Today, 8(4), 795–803.
Abubakar, A. R., & Haque, M. (2020). Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy and Bioallied Sciences, 12(1), 1.
Al-Rimawi, F., Abu-Lafi, S., Abbadi, J., Alamarneh, A. A., Sawahreh, R. A., & Odeh, I. (2017). Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. African Journal of Traditional, Complementary and Alternative Medicines, 14(2), 130-141.
Al-Rimawi, F., Alakhras, F., Al-Zereini, W. A., Aldal'in, H. K., Abu-Lafi, S., Al-Mazaideh, G. M., & Salman, H. J. A. (2018). HPLC analysis of chemical composition of selected jordanian medicinal plants and their bioactive properties. Original Journal of Chemistry, 34(5), 2397-2403.
Anusha, S., Madhu, M. L., Seethalakshmy, S., & Nair, S. A. ANTIOXIDANT AND ANTIMICROBIAL ACTIVITIES OF METHANOLIC LEAF EXTRACTS OF Coriandrum sativum AND Eryngium foetidum L. International Journal of Research and Engineering, 2(8), 28-32.
Azwanida, N. (2015). A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants, 4(196), 2167-0412.
Biglari, F., AlKarkhi, A. F., & Easa, A. M. (2008). Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food chemistry, 107(4), 1636-1641.
Blois, M. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181(4617), 1199-1200.
Brand-Williams, W., Cuvelier, M.-E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology, 28(1), 25-30.
Cannel, R. J. (1998). Methods in Biotechnology. Natural Products Isolation. Humana Press, Totowa, New Jersey, 4, 1-285.
Chandira, R. M., & Jaykar, B. (2013). Extraction, pharmacological evaluation and formulation of selected medicinal herbs for antidiabetic activity. International Journal of Pharmacy Teaching & Practices, 4(1), 458-482.
Dalukdeniya, D., & Rathnayaka, R. (2017). Comparative study on antibacterial and selected antioxidant activities of different Eryngium Foetidum extracts. Journal of Applied Life Sciences International, 12(4), 1-7.
Dawilai, S., Muangnoi, C., Praengamthanachoti, P., & Tuntipopipat, S. (2013). Anti-inflammatory activity of bioaccessible fraction from Eryngium foetidum leaves. BioMed research international, 2013, 1-8.
Devgun, M., Nanda, A., Ansari, S., & Swamy, S. (2010). Recent techniques for extraction of natural products. Research Journal of Pharmacy Technology, 3(3), 644-649.
Eyoum Bille, B., & Nguepi, E. (2016). In vitro and in vivo anti-Helicobacter activities of Eryngium foetidum (Apiaceae), Bidens pilosa (Asteraceae), and Galinsoga ciliata (Asteraceae) against Helicobacter pylori. BioMed research international, 2016, 1-7.
Firuzi, O., Lacanna, A., Petrucci, R., Marrosu, G., & Saso, L. (2005). Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochimica et Biophysica Acta -General Subjects, 1721(1-3), 174-184.
Garcia, M., Saenz, M., Gomez, M., & Fernandez, M. (1999). Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytotherapy research, 13(1), 78-80.
Gayathri, V., & Kiruba, D. (2014). Preliminary phytochemical analysis of leaf powder extracts of Psidium guajava L. International Journal of Pharmacognosy and Phytochemical Research, 6(2), 332-334.
Gliszczyńska-Świgło, A. (2006). Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food chemistry, 96(1), 131-136.
Handa, S. S., Khanuja, S. P. S., Longo, G., & Rakesh, D. D. (2008). Extraction technologies for medicinal and aromatic plants: Earth, Environmental and Marine Sciences and Technologies.
Hanif, M. A., Nisar, S., Khan, G. S., Mushtaq, Z., & Zubair, M. (2019). Essential oils. In Essential oil research (pp. 3-17): Springer.
Kokilananthan, S., Bulugahapitiya, V., Manawadu, H., & Gangabadage, C. (2022). Comparative Evaluation of Different Extraction Techniques on Phytochemicals and Antioxidant Activity of Psidium Guajava L. Trop J Nat Prod Res, 6(4), 552-557.
Kokilananthan, S., Bulugahapitiya, V. P., Gangabadage, C. S., & Manawadu, H. (2021). Comparative accounts on proximate and phytochemical compositions and antioxidant properties of Garcinia quaesita and Garcinia zeylanica. International Journal of Minor Fruits, Medicinal and Aromatic Plants, 7(2), 59-67.
Kokilananthan, S., Vajira, P. B., Gangabadage, C. S., & Harshi, M. (2020). Comparative account on antioxidant properties, proximate and phytochemical compositions of seven guava varieties grown in Sri Lanka. Journal of Agriculture and Value Addition, 3(2), 1-16.
Kokilananthan, S., Vajira, P. B., Gangabadage, C. S., & Harshi, M. (2022). Effect of extraction techniques on phytochemicals and antioxidants activity of Garcinia quaesita leaves. Advances in Technology, 2(1), 18-30.
Li, H.-B., Jiang, Y., Wong, C.-C., Cheng, K.-W., & Chen, F. (2007). Evaluation of two methods for the extraction of antioxidants from medicinal plants. Analytical and bioanalytical chemistry, 388(2), 483-488.
Lingaraju, D., Sudarshana, M., Mahendra, C., & Rao, K. P. (2016). Phytochemical screening and antimicrobial activity of leaf extracts of Eryngium foetidum L.(Apiaceae). Indo American Journal of Pharmaceutical Research, 6(2), 4339-4344.
Malik, T., Pandey, D. K., Roy, P., & Okram, A. (2016). Evaluation of phytochemicals, antioxidant, antibacterial and antidiabetic potential of Alpinia galanga and Eryngium foetidum Plants of Manipur (India). Pharmacognosy Journal, 8(5).
Manousi, N., Sarakatsianos, I., & Samanidou, V. (2019). Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering tools in the beverage industry (pp. 283-314): Elsevier.
Mtewa, A. G., Deyno, S., Kasali, F. M., Annu, A., & Sesaazi, D. C. (2018). General extraction, isolation and characterization techniques in drug discovery: A review. Int. J. Sci. Basic Appl. Res, 38(1), 10-24.
Okon, J., Edet, E., Esenowo, G., & Umoh, N. (2013). Phytochemical screening, analgesic and antiinflammatory properties and Median lethal dose of ethanol leaf extract of wild species of Eryngium foetidum L. on Albino rats. International Journal of Modern Biology and Medicine, 3(2), 69-77.
Pandey, A., & Tripathi, S. (2014). Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry, 2(5).
Promkum, C., Butryee, C., Tuntipopipat, S., & Kupradinun, P. (2012). Anticlastogenic effect of Eryngium foetidum L. assessed by erythrocyte micronucleus assay. Asian Pacific Journal of Cancer Prevention, 13(7), 3343-3347.
Sathyanarayanan, S., Muniyandi, K., George, E., Sivaraj, D., Sasidharan, S. P., & Thangaraj, P. (2017). Chemical profiling of Pterolobium hexapetalum leaves by HPLC analysis and its productive wound healing activities in rats. Biomedicine and Pharmacotherapy, 95, 287-297.
Shanthirasekaram, K., Bulugahapitiya, V., Manawadu, H., & Gangabadage, C. (2021). Phytochemicals and antioxidant properties of the leaves of wild guava varieties grown in Sri Lanka. Journal of Science, 12(2), 33-46.
Stratakos, A. C., & Koidis, A. (2016). Methods for extracting essential oils. In Essential oils in food preservation, flavor and safety (pp. 31-38): Elsevier.
Wadood, A., Ghufran, M., Jamal, S. B., Naeem, M., Khan, A., & Ghaffar, R. (2013). Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem, 2(4), 1-4.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Malaysian Journal of Science

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





