A SENSITIVE SPECTROPHOTOMETRIC METHOD FOR TRACE AMOUNTS DETERMINATION OF PROMETHAZINE IN DRUG FORMULATIONS VIA ION PAIR COMPLEX FORMATION
Main Article Content
Abstract
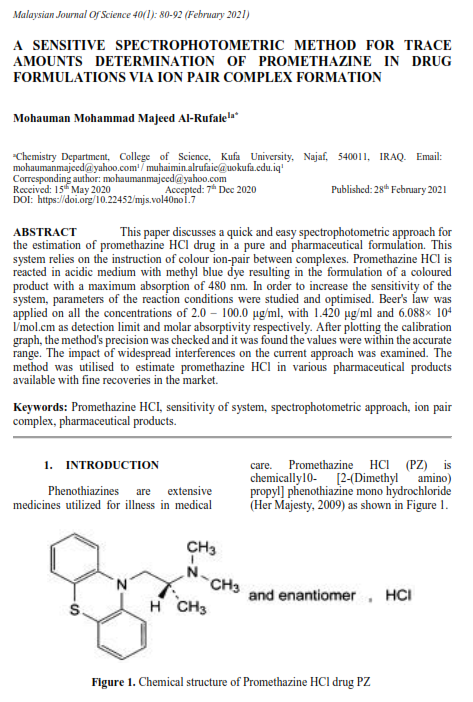
ABSTRACT This paper discusses a quick and easy spectrophotometric approach for the estimation of promethazine HCl drug in a pure and pharmaceutical formulation. This system relies on the instruction of colour ion-pair between complexes. Promethazine HCl is reacted in acidic medium with methyl blue dye resulting in the formulation of a coloured product with a maximum absorption of 480 nm. In order to increase the sensitivity of the system, parameters of the reaction conditions were studied and optimised. Beer's law was applied on all the concentrations of 2.0 – 100.0 μg/ml, with 1.420 μg/ml and 6.088× 104 l/mol.cm as detection limit and molar absorptivity respectively. After plotting the calibration graph, the method's precision was checked and it was found the values were within the accurate range. The impact of widespread interferences on the current approach was examined. The method was utilised to estimate promethazine HCl in various pharmaceutical products available with fine recoveries in the market.
Downloads
Article Details
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).
References
936.
Al-Rufaie, M.M., Al-Sharefy, A.N. & Kathem, K.H. (2013): New spectrophotometric method for the determination chlorpromazine hydrochloride in pharmaceutical preparations by using oxidative coupling reaction. Inter. J. of Uni. Pharmacy and Bio Sciences 2(4),
19-26.
Al-Rufaie, M. M. (2016): New spectrophotometric method for the determination of Sulfamethoxazole drug. W. J. of pharmacy and pharmaceutical sciences. 5(3), 172-
180.
Al-Rufaie, M.M. (2016): Modern kinetic spectrophotometric procedure for estimation of furosemide drug as bulk form and in pharmaceuticals preparations. Curr. Issues Pharm. Med. Sci. 29(4), 184-190.
Al-Rufaie, M.M., Al-labban, H. M.Y. & Salih, N.S. (2017): Reduction and assessment of chloramphenicol antibiotic as pure from and in various kinds of pharmaceuticals by utilizing spectrophotometric approach. Iran. J. of Org. Chem.
9(2), 2087-2092.
AL-Ward, H. S. (2005): Spectrophotometric micro determination of promethazine hydrochloride in pharmaceutical preparations via oxidative coupling reaction with sulphanilamide and
in the presence of ferric chloride. Journal of Um-Salama for Science
2, 110-119.
Ahmed K. H., Bahruddin, A. Sulaiman S.
& Rohana A. (2011): Ionophore- Based Potentiometric Sensors for the Flow-Injection Ionophore- Based Potentiometric Sensors for the Flow-Injection "Determination of promethazine Hydrochloride in Pharmaceutical Formulations and Human Urine.J. Sensors. 11(1),
1028-1132.
Basavaiah K. (2004): Determination of some psychotropic phenothiazine drugs by charge-transfer complication reaction with chloranil acid.IIFarmaco 59, 315-
322.
Baxter, R.I., Svehla, G. Kerr, B. & Woolfson A.D. (1984): Determination of promethazine by anodic differential pulse voltammetry. Anal. Chim. Acta 64,
171-176.
Calatayud, J. M. & Sancho T.G. (1992): Spectrophotometric determination of promethazine by flow-injection analysis and oxidation by Ce IV. J. Pharm. Biomed. Anal. 10, 7-13.
Calatayud, J. M, Sarrion, S.N., Sampedro A.S., & Benito C.G. (1992): Determination of promethazine hydrochloride with bromophenol blue by a turbid metric method and flow injection analysis. Microchem. J.45, 129-137.
Daniel, D. & Gutz I.G.R. (2003): Flow injection spectroelectro analytical method for the determination of promethazine hydrochloride in pharmaceutical preparations. Anal. Chim. Acta.494 (1-2), 215-222.
Hanaa, K.A.T., Al-Rufaie, M.M. & Zahraa, Y.M. (2018): Spectrophotometric determination of metoclopramide medicine in bulk form and in pharmaceuticals using orcinol as reagent. Ovidius University Annals of Chemistry 29 (2), 85-89.
Hemn, A.Q. & Nabil, A. F. (2017): Spectrophotometric Determination of Promethazine Hydrochloride in Pure and Pharmaceutical Dosage Forms. ZANCO J. of Pure and Applied Sci.29 (s4), 107,118.
Howard, C.A., Loyd, V. A. & Nicholas, G.
P. (1990): Pharmaceutical dosage forms and Drug Delivery Systems, Lippincott Williams & Wilkins Publishers, p.51.
Ibrahim, E.A., Issa, A.S., Abdel salam, M.A. & Mahrous, M.S. (1983): The use of chloranil for spectrophotometric determination of some tranquillizers and antidepressants. Talanta 30, 531-
540.
Jawad, A.A. & K. H. Kathem (2013): Spectrophotometric determination of metoclopramide hydrochloride in bulk and pharmaceutical preparations by diazotization- coupling reaction. Inter. J. of Pharmacy and Pharmaceutical Sciences, 5, 294-299.
Khaleda, H. A. & Zainab, W. A. (2011): Construction of promethazine Hydrochloride Selective Electrode in a PVC matrix Membrane. J. of AL-Nahrain University- Sci. 14(4),
11-17.
Kubacak, P., Mikus, P., Valaskova I. & Havranek, E. (2005): Determination of promethazine hydrochloride in pharmaceuticals by capillary isotachophoresis.
Methods Findings Exp. Clin. Pharmacol. 27, 529-531.
Muijselaar, P.G.H.M., Claessens H.A. & Cramers, C.A. (1996): Determination of structurally related Phenothiazines by capillary zone electrophoresis and micellar electro kinetic chromatography. J. Chromatogr. A 735, 395-401.
Nabil, S. N., Shahbaz, A. M.S. & Bashaer, A. A. (2008): Preparation and Potentiometric Study of promethazine Hydrochloride Selective Electrodes and Their Use in Determining Some Drugs. Turk J Chem.32, 539-544.
Najim, A. S., Nief, R.A. & Mona, I.I. (2006): spectrophotometric determination of promethazine hydrochloride VIA oxidative coupling reaction with sulfanilic acid. App.Sci.3, 1-8.
Nina, A. & Zahra, R. (2012): Extractive Spectrophotometric determination of Ketoconazole, Clotrimazole AND Fluconazole by ion –pair complex formation with bromothymol blue and picric acid. J. Chil. Chem. Soc.57 (2), 1104-
1111.
Pena, L. D. L., Gomez-Hens, A. & Perez- Bendito, D. (1993): Kinetic fluorimetric determination of promethazine by a stopped- flow mixing technique, J. Pharm. Biomed. Anal.11, 893-900.
Regulska, E., Tarasiewicz, M. & Puzanowska T.H. (2002): Extractive-spectrophotometric determination of some Phenothiazines with dipicrylamine and picric acid. J. pharm.Biomed.Anal.27, 335-342.
Salah, M. I. & Yoong, C.S. (1993): Chromatographic Method for Separation and Determination of Promethazine HCl in Pharmaceutical doses.J.Phys.Sci.4,
55-60.
Saleh, O., El-Azzouny, A., Aboul-Enein, H. & Badawy, A. M. (20090: A Validated HPLC Method for Separation and Determination of Promethazine Enantiomers in Pharmaceutical Formulations. Drug Dev. Ind. Pharm.35, 19-23.
Shadi Asadollahi, M.D., Kamran Heidari, M.D., Reza Vafaee, M.D., Mohammad Mahdi, M.D., Afshin Amini, M.D. & Ali Shahrami, M.D. (2000): Promethazine Plus Sumatriptan in the Treatment of Migraine: A Randomized Clinical Trial. Wiley Periodicals, Inc. p.12259.
Sultan, S. M. & Suliman, F. (1992): Application of super modified simplex optimization to the flow- injection spectrophotometric determination of promethazine hydrochloride in drug formulations. Anal.Sci.8, 841-849.
Sultan, S.M. Hassan, Y.A.M. & Abulkibash, A.M. (2003): Chemiluminescence assay of promethazine hydrochloride using
acidic permanganate employing flow injection mode operated with syringe and peristaltic pumps. Talanta 59(6) (2003) 1073-1079.
Taylor, G. & Houston, J.B. (1982): Simultaneous determination of promethazine and two of its circulating metabolites by high performance liquid chromatography. J. Chromatogr. B
23, 194-201.
Tepper, S.J., Rapoport, A.M. & Sheftell, F.D. (2002): mechanism of action of the 5-HT1D/1B receptor agonist. Arch Neurol. 59 (7), 1084-
1089.
The British Pharmacopoeia (2009), Her Majesty’s Stationary Office. London, pp.5010, 5013, 9844,
9848.
Theia'a, N. A. Nie, R.A. & Mona, I.I. (2006): Spectrophotometric Determination of Promethazine hydrochloride via Oxidative Coupling Reaction with Sulfanilic Acid. J. of P. & App.Sci. 22, 321-
329.
Yongnian, LW.N. & Kokot, S. (2001): Voltammetric determination of chlorpromazine hydrochloride and promethazine hydrochloride with the use of multivariate calibration. Anal. Chim. Acta 439, 159-165.